
The environmental nanogeosciences involve the study of environmental materials (such as soil, water-solid interfaces, organic and inorganic particles) and nano scale processes that affect chemical reactivity, mobility, and availability to biota. These material can be derived from naturally occuring processes or from anthrophogenic acitivities (e.g. engineered nanomaterials, incidentially produced from human activities).
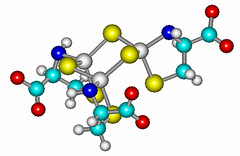
The Hsu-Kim group is actively involved in the study of the environmental implications of naturally occuring and anthropogenic nanomaterials. Our group has studied the major processes that influence the fate of metal and mineral-based nanomaterials. This work has involved a variety of metallic and mineral materials, including metallic silver, zinc oxide, cerium dioxide, and metal sulfides. Currently we are studying biogenic nanoparticles such as bacterial extracellular vesicles. An overarching theme of this work is that the the interactions of these materials with their surroundings depend on a variety of intrinsic properties of the material (e.g., surface composition, structure) that can be linked to relevant environmental processes for trace elements such as mobility, redox reactivity and bioavailability.
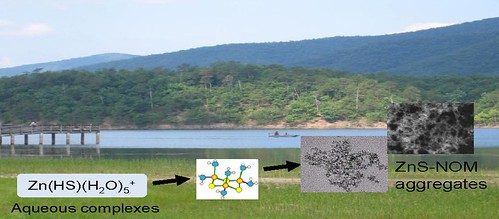
We closely collaborate with other researchers who are part of the Center for Environmental Implications of NanoTechnology (CEINT), headquartered at Duke. This work is currently supported by the U.S. National Science Foundation.

Selected publications:
ES&T Special Issue: Nanoscale Organic Matter Interactions and Feature Article (2011);
Metal sulfides: ES&T (2008, 2009, 2011); Chemical Geology (2012); JCIS (2010);
Metallic silver: ES&T (2012), (2012), (2014), Ecotoxicology (2016), Nanotoxicology (2015)
Soluble Metal Oxides and dissolution kinetics: ESPI (2014), ES&T (2015) (2017)
Effects of nanoparticles on aquatic passive samplers: ES&T (2015)
Selected publications:
Rivera, N.A., F.T. Ling, Z. Jin, A. Pattammattel, H. Yan, Y.S. Chu, C.A. Peters, and H. Hsu-Kim. Nanoscale heterogeneity of arsenic and selenium species in coal fly ash particles: analysis using enhanced spectroscopic imaging and speciation techniques. Environmental Science: Nano 10, 768–77. https://doi.org/10.1039/d2en01056a
McMillan, H.M.; Rogers, N.; Wadle, A.; Hsu-Kim, H.; Wiesner, M.R.; Hendren, C.O. (2021) Microbial vesicle-mediated communication: convergence to understand interactions within and between domains of life. Environmental Science: Processes & Impacts. 23, 664-677. DOI: 10.1039/D1EM00022E
Geitner, N.; Cooper, J.; Avellan, A.; Castellon, B.; Perrotta, B.; Bossa, N.; Simonin, M.; Anderson, S.; Inoue, S.; Hochella, M.; Richardson, C.; Bernhardt, E.; Lowry, G.; Ferguson, P.L.; Matson, C.; King, R.; Unrine, J.; Wiesner, M.R.; Hsu-Kim, H. (2018). Size-Based Differential Transport, Uptake, and Mass Distribution of Ceria (CeO2) Nanoparticles in Wetland Mesocosms. Environ. Sci. & Technol. 52, 9768-9776. DOI: 10.1021/acs.est.8b02040
Jiang, C.; Castellon, B.T.; Matson, C.W.; Aiken, G.R.; Hsu-Kim, H. (2017). Relative contributions of copper oxide nanoparticles and dissolved copper to Cu uptake kinetics of Gulf killifish (Fundulus grandis) embryos. Environ. Sci. & Technol. 51, 1395-1404 . DOI:10.1021/acs.est.6b04672
Jiang, C.; Aiken, G.R.; Hsu-Kim, H. (2015). Effects of Natural Organic Matter Properties on the Dissolution Kinetics of Zinc Oxide Nanoparticles. Environ. Sci. & Technol. 49(19), 11476-11484. DOI: 10.1021/acs.est.5b02406
Pham, A. L.-T.; Morris, A., Zhang, T., Ticknor, J.; Levard, C.; Hsu-Kim, H. (2014). Precipitation of Nanoscale Mercuric Sulfides in the Presence of Natural Organic Matter: Structural Properties, Aggregation, and Biotransformation. Geochim. Cosmochim. Acta. 133, 204-215. DOI: 10.1016/j.gca.2014.02.027.
Zhang, T.; Kucharzyk, K.H.; Kim, B.; Deshusses, M.A.; Hsu-Kim, H. (2014). Net methylation of mercury in estuarine sediment microcosms amended with dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ. Sci. & Technol. 16, 9133-9141. DOI: 10.1021/es500336j.
Aiken G.R., Hsu-Kim H., Ryan J.N. (2011). Influence of dissolved organic matter for the environmental fate of metals, nanoparticles, and colloids. Envir. Sci & Technol. 45, 3196–3201. [publication]
